WHO clears vaccine as first shot against mpox
By REUTERS
What you need to know:
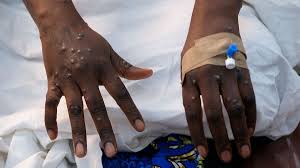
- Mpox, a viral infection that spreads through close contact and is usually mild but can kill, was declared a public emergency of international concern by the WHO last month.
The World Health Organization said on Friday it had cleared Bavarian Nordic’s BAVA.CO mpox vaccine, the first such shot to be approved by the agency for containing the spread of the disease in badly hit African countries.
The approval, known as a prequalification, comes as a new type of the virus spreads from the Democratic Republic of Congo, where the current outbreak began in early 2023, to several neighboring countries.
“This first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in the future,” WHO Director-General Dr Tedros Adhanom Ghebreyesus said.
The prequalification paves the way for developing countries to access the shot as most of these nations do not have the resources to do rigorous checks into the safety and efficacy of vaccines. U.N. agencies also rely on the process before buying medical products.
The Bavarian Nordic vaccine, known as Jynneos in the United States, was originally approved as a smallpox shot. Some doses have been donated in Congo, where the first round of inoculations is due to begin in early October.
“The evidence we have now is that it is important we take advantage of it (the vaccine) to protect our population,” Dimie Ogoina, chair of the WHO’s mpox emergency committee, had said before the approval.
He however stressed that vaccines were not a “magic bullet” and other public health measures like testing and contact tracing were important. The public also needed to be informed that some unknowns remain, including how long protection from mpox lasts, he said.
“OFF-LABEL” USE IN CHILDREN
Bavarian Nordic said the vaccine was cleared for immunization against smallpox, mpox, and related orthopoxvirus infections and disease in adults 18 years of age and older.
The Danish biotech company has said it can supply 13 million doses the vaccine by the end of 2025.
The vaccine may, however, be used “off-label” in infants, children and adolescents, and in pregnant and immuno-compromised people in outbreak settings where the benefits of vaccination outweigh the potential risks.
The DRC has said it will not vaccinate kids in the first phase of the inoculation campaign.
A second vaccine made by Japan’s K M Biologics, which is also under review by the WHO, can be used for children, although it is not available outside of Japan and requires special needles to administer.